There will be major changes to Panadol on shelves ahead of a decision by the Therapeutic Goods Administration (TGA) to change the packaging of paracetamol-containing products.
The TGA announced a series of changes to paracetamol packaging last year, stating that the maximum size of packs available for general sale will fall from 20 capsules to 16.
The change will come into effect from February 2025 in supermarkets and convenience stores, among others.
However, the size of packs for sale in pharmacies without the supervision of a pharmacist will also be reduced by half, from 100 tablets or capsules to 50.
Packs containing up to 100 tablets or capsules are only available under the supervision of a pharmacist, while capsules and tablets are only available in blister packs.
The decision took into account an independent expert report commissioned by the TGA, which found that the number of deliberate self-poisonings with paracetamol has increased over the past decade, especially among adolescents and young adults.
It also found that paracetamol is the most commonly used drug in overdoses among young Australians, with the figure holding steady at 50 per cent.
“To further minimize the harm caused by paracetamol overdose, the TGA encourages retailers such as supermarkets to limit sales to one pack at a time,” the TGA announcement said.
From February 2025, the maximum size of Panadol packs available for general sale in supermarkets and convenience stores will be reduced from 20 tablets to 16 tablets.
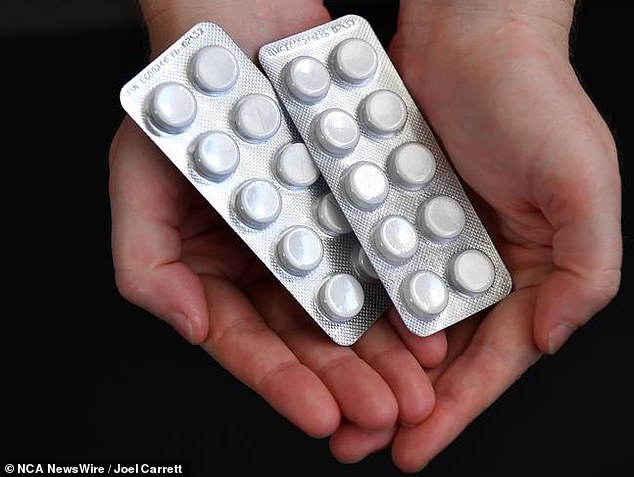
At pharmacies without pharmacist supervision, the pack size will decrease from 100 tablets or capsules to 50 tablets.
‘The TGA also encourages consumers not to have paracetamol at home, but to store paracetamol and other medicines properly.
‘To give producers and retailers sufficient time to adapt, the decision will come into effect from February 1, 2025.’
Following the decision, Panadol has already started implementing the changes ahead of the February 2025 deadline.
Haleon (the makers of Panadol) pain head Elena Pintado said that after the changes there are no changes in the ‘suitability profile and efficacy of acetaminophen for pain relief when used as directed’.

Painaustralia ambassador Sophie Scott (pictured) said paracetamol remains ‘effective’ when used as directed
“Our focus is always on the health of Australians and facilitating responsible self-care. “As the makers of Panadol, Australia’s best-known pain relief brand, we take our responsibility very seriously to ensure Australians can continue to access Panadol without disruption once these changes come into effect,” Ms Pintado said.
Painaustralia ambassador Sophie Scott said paracetamol remains ‘effective’ when used as directed.
‘Everyday pain affects a large number of Australians and paracetamol is widely used to treat it. Paracetamol is effective for daily pain relief when used as directed,” Ms Scott said.
‘It is important that we follow the instructions on the package and consult a healthcare provider if our pain persists.’